Healthcare professionals have chosen Lantus® for over 18 years1
LANTUS® DEMONSTRATED SAFETY AND IMPROVED GLYCEMIC CONTROL VS NPH1-3
Lantus® was evaluated for 52 weeks in adults with type 2 diabetes poorly controlled with OADs
Mean A1C Levels

- Mean A1C reduction was 0.5% with Lantus® and 0.4% with NPH at 52 weeks
NPH, Neutral protamine Hagedorn insulin: OADs, oral anti-diabetic drugs
Insulin dose and reductions in FPG during a 52-week study

- Lantus® dose at 52 weeks: 26 Units (range 3 Units to 120 Units)
- An appropriate dose and proper titration helped many patients achieve glycemic targets
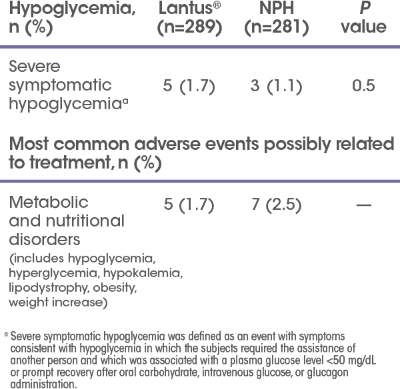
Safety results
.png 400w, /.imaging/webp/sanofi-platform/img-w500/dam/lantus.com-hcp/Homepage/lantus-hcp-103118-efficacy-safety-m2x--1-.png/jcr:content/lantus-hcp-103118-efficacy-safety-m2x%20(1).png 500w)
Study Design
A 52-week randomized study (N=570) designed to compare efficacy and safety of Lantus® plus OADs vs NPH plus OADs in patients with type 2 diabetes poorly controlled with OADs. Patients were randomized to either Lantus® (n=289) or NPH (n=281) at bedtime to reach a target FPG of <120 mg/dL. OADs were continued. Initial insulin dose and titration schedule were left to the discretion of the individual investigators. Primary endpoint was change in A1C.
IN A TREAT-TO-TARGET PEDIATRIC TRIAL WITH NPH1,4,5
In a pediatric clinical study, children and adolescents with T1DM had a higher incidence of severe symptomatic hypoglycemia in the 2 treatment groups (Lantus® or NPH) compared to adult trials with type 1 diabetes.
- Baseline A1C was 8.48% and 8.81% for the Lantus® and NPH groups, respectively
- The relative change in A1C at 28 weeks was +0.28% for the Lantus® group (n=174) and +0.27% for the NPH group (n=175)
Once-daily pediatric dosing
In the same pediatric trial
- 35% of patients on NPH needed to inject twice as often as patients on once-daily Lantus® in order to maintain comparable A1C levels
Safety results
Fewer pediatric patients on Lantus® experienced severe hypoglycemia

Most common adverse events possibly related to treatment for the Lantus® and NPH groups (P=NS)
- Injection site reactions (includes atrophy, hemorrhage, mass, pain, reaction): Lantus® 5.2%; NPH 2.9%
- Metabolic and nutritional disorders (includes hypoglycemic reaction, lipodystrophy): Lantus® 2.3%; NPH 4.0%
Study Design
A 28-week, randomized, open-label, multicenter study of 349 patients with type 1 diabetes (aged 6-15) who received once-daily Lantus® (n=174) or once- or twice-daily NPH (n=175) in combination with regular human insulin as the mealtime insulin. The primary efficacy measure was mean change in A1C from baseline.
aSymptomatic hypoglycemia was defined as any event with clinical symptoms that could be confirmed by BG level <50 mg/dL.
bSevere hypoglycemia was defined as an event with symptoms consistent with hypoglycemia in which the subjects required the assistance of another person and which was associated with a BG level <50 mg/dL or prompt recovery after oral carbohydrate, intravenous glucose, or glucagon administration. This definition is consistent with that used in the Diabetes Control and Complications Trial.

Go to interactive dosing calculator
Indication and Use
LANTUS is a long-acting human insulin analog indicated to improve glycemic control in adult and pediatric patients with diabetes mellitus.
Limitations of Use
Lantus is not recommended for the treatment of diabetic ketoacidosis.
Important Safety Information for Lantus (insulin glargine injection) 100 Units/mL
Important Safety Information for Lantus (insulin glargine injection) 100 Units/mL
Contraindications
Lantus is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin glargine or any of the excipients in LANTUS.
Warnings and Precautions
Insulin pens, needles, or syringes must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimen only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant drugs.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Do not dilute or mix Lantus with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Lantus via an insulin pump or intravenously because severe hypoglycemia can occur.
Hypoglycemia is the most common adverse reaction of insulin therapy, including Lantus, and may be life-threatening.
Hypoglycemia due to medication errors, such as accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Severe life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Lantus, treat and monitor until symptoms resolve.
A reduction in the Lantus dose may be required in patients with renal or hepatic impairment.
As with all insulins, Lantus use can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Lantus include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema and weight gain.
Important Safety Information for Lantus SoloSTAR
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying the pen: otherwise they may not get the correct amount of insulin, which may affect their blood glucose.
Click here for Full Prescribing Information for Lantus.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms for your Patients:
Insulins Valyou Savings Program: This savings program is not insurance.
For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance.
For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Reference:
1. Lantus® Prescribing Information.
2. Data on file (CSR F1998CLN0004, June 1999).
3. Yki-Järvinen H, Ziemen M, Dressler A; HOE 901/3002 Study Group. Diabetes Care. 2000;23(8):1130-1136.
4. Schober E, Schoenle E, VanDyk J, Wenicke-Panten K. J Pediatr Endocrinol Metab. 2002;15:369-376.
5. Data on file. (Schrober CSR), Sanofi US.
All registered trademarks cited are property of their respective owners.
Important Safety Information for Lantus (insulin glargine injection) 100 Units/mL
Contraindications
Lantus is contraindicated during episodes of hypoglycemia and in patients with hypersensitivity to insulin glargine or any of the excipients in LANTUS.
Warnings and Precautions
Insulin pens, needles, or syringes must never be shared between patients. Do NOT reuse needles.
Monitor blood glucose in all patients treated with insulin. Modify insulin regimen only under medical supervision. Changes in insulin regimen including, strength, manufacturer, type, injection site or method of administration may result in the need for a change in insulin dose or an adjustment in concomitant drugs.
Repeated insulin injections into areas of lipodystrophy or localized cutaneous amyloidosis may result in hyperglycemia; sudden change in the injection site (to unaffected area) has been reported to result in hypoglycemia. Advise patients to rotate injection site to unaffected areas and closely monitor for hypoglycemia.
Do not dilute or mix Lantus with any other insulin or solution. If mixed or diluted, the solution may become cloudy, and the onset of action/time to peak effect may be altered in an unpredictable manner. Do not administer Lantus via an insulin pump or intravenously because severe hypoglycemia can occur.
Hypoglycemia is the most common adverse reaction of insulin therapy, including Lantus, and may be life-threatening.
Hypoglycemia due to medication errors, such as accidental mix-ups between basal insulin products and other insulins, particularly rapid-acting insulins, have been reported. Patients should be instructed to always verify the insulin label before each injection.
Severe life-threatening, generalized allergy, including anaphylaxis, can occur. Discontinue Lantus, treat and monitor until symptoms resolve.
A reduction in the Lantus dose may be required in patients with renal or hepatic impairment.
As with all insulins, Lantus use can lead to life-threatening hypokalemia. Untreated hypokalemia may cause respiratory paralysis, ventricular arrhythmia, and death. Closely monitor potassium levels in patients at risk of hypokalemia and treat if indicated.
Fluid retention, which may lead to or exacerbate heart failure, can occur with concomitant use of thiazolidinediones (TZDs) with insulin. These patients should be observed for signs and symptoms of heart failure. If heart failure occurs, dosage reduction or discontinuation of TZD must be considered.
Drug Interactions
Certain drugs may affect glucose metabolism, requiring insulin dose adjustment and close monitoring of blood glucose. The signs of hypoglycemia may be reduced in patients taking anti-adrenergic drugs (e.g., beta-blockers, clonidine, guanethidine, and reserpine).
Adverse Reactions
Adverse reactions commonly associated with Lantus include hypoglycemia, allergic reactions, injection site reactions, lipodystrophy, pruritus, rash, edema and weight gain.
Important Safety Information for Lantus SoloSTAR
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. To help ensure an accurate dose each time, patients should follow all steps in the Instruction Leaflet accompanying the pen: otherwise they may not get the correct amount of insulin, which may affect their blood glucose.
Click here for Full Prescribing Information for Lantus.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi's commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms for your Patients:
Insulins Valyou Savings Program: This savings program is not insurance.
For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance.
For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Reference:
1. Lantus® Prescribing Information.
2. Data on file (CSR F1998CLN0004, June 1999).
3. Yki-Järvinen H, Ziemen M, Dressler A; HOE 901/3002 Study Group. Diabetes Care. 2000;23(8):1130-1136.
4. Schober E, Schoenle E, VanDyk J, Wenicke-Panten K. J Pediatr Endocrinol Metab. 2002;15:369-376.
5. Data on file. (Schrober CSR), Sanofi US.
All registered trademarks cited are property of their respective owners.

