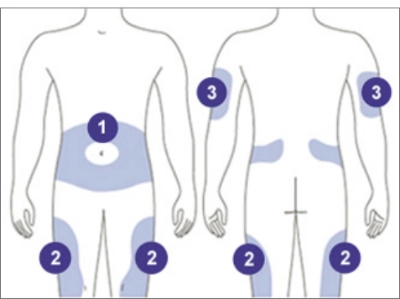
INSULIN INJECTION SITES: CHOOSE THE BEST SITE FOR YOU
Using Lantus doesn’t have to be difficult, but it does take practice. Your doctor or a member of your healthcare team will show you how.
Important Information For Use
- Inject into the less sensitive layer of fatty tissue just under the skin
- It should not be injected into the muscle
- Rotate your injection sites with each dose to reduce your risk of getting lipodystrophy (pitted or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
- Do not share needles, pens, or syringes with others. Do NOT reuse needles.
-
THE STOMACH
Except for a 2-inch circle around the navel
-
THE TOP AND OUTER THIGHS
Avoid administering too close to the bony area above the knee
-
THE OUTER, UPPER ARMS
Use the outer back area of the upper arm where there is fatty tissue
HOW TO USE THE LANTUS SOLOSTAR INSULIN PEN
Now that you and your doctor have decided Lantus is right for you, watch this step-by-step video to learn how to use the Lantus SoloStar pen. But always follow your healthcare provider’s instructions.
Rotate your injection sites as instructed by your healthcare provider to reduce your risk of getting lipodystrophy (pitted or thickened skin) and localized cutaneous amyloidosis (skin with lumps). Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
- LANTUS is not for use to treat diabetic ketoacidosis
- small, thin needles
- large print dosing window
- dial-in dose
- push-button injection
- Checking the insulin
- Attaching the needle
- Performing a safety test
- Selecting the dose
- Injecting the dose
- Removing and discarding the needle
- allow pen to freeze
- Put it next to the freezer compartment or next to a freezer pack
- Do not use a SoloSTAR® pen after the expiration date.
- Protect your SoloSTAR® pen from light.
- Discard your used SoloSTAR® pen as required by your local authorities.
- Protect your SoloSTAR® pen from dust and dirt.
- You can clean the outside of your SoloSTAR® pen by wiping it with a damp cloth.
- Do not soak, wash, or lubricate the pen as this may damage it.
- Your SoloSTAR® pen is designed to work accurately and safely. It should be handled with care. Avoid situations where the SoloSTAR® pen might be damaged. If you are concerned that your SoloSTAR® pen may be damaged, use a new one.
- If you have any further questions about the Lantus® SoloSTAR® pen or managing your diabetes, be sure to speak to your doctor, nurse, or pharmacist.
- LANTUS is not for use to treat diabetic ketoacidosis
- Shortness of breath
- Swelling of your ankles or feet
- Sudden weight gain
- A rash over your whole body
- Trouble breathing
- A fast heartbeat
- Sweating
- Swelling of your face, tongue, or throat
- Shortness of breath
- Extreme drowsiness, dizziness, or confusion
SUPER: These instructions do not replace the guidance of your doctor or the instructions for use that accompanies the Lantus (insulin glargine injection) 100 Units/mL SoloStar pen.
People who are blind or have vision problems should not use Lantus SoloStar prefilled pen without the help from a person trained to use Lantus SoloStar prefilled pen.
Laura: Hi, my name is Laura. I’d like to take a few minutes to tell you what you need to know about starting Lantus® (insulin glargine injection) 100 Units/mL using the SoloSTAR® pen. You’ve taken an important step by adding Lantus® to your diabetes treatment plan. One dose of Lantus® at the same time each day works all day and all night.
Laura: Prescription LANTUS is a long-acting man-made-insulin used to control high blood sugar in adults and children with diabetes mellitus.
Laura: Important Safety Information for Lantus (insulin glargine injection) 100 Units/mL. Do not take Lantus® during episodes of low blood sugar or if you are allergic to insulin or any of the inactive ingredients in Lantus®.
SUPER: The Lantus® SoloSTAR® pen delivers the dose you dial time after time.
Laura: The Lantus® SoloSTAR® pen delivers the dose you dial time after time. Today, I’ll be demonstrating the correct way to use the Lantus® SoloSTAR® pen.
SUPER: The Lantus® SoloSTAR® pen features:
Laura: The Lantus® SoloSTAR® pen can fit into your daily routine. It features small, thin needles, a large print dosing window, dial-in dose, and push-button injection.
SUPER: Do not share needles, insulin pens, or syringes with others. Do NOT reuse needles.
Laura:Do not share needles, insulin pens, or syringes with others. Do NOT reuse needles.
Laura:To demonstrate how to correctly use the SoloSTAR® pen, I’ll be taking you through these 6 steps:
Super: WHAT WE’LL COVER:
STEP 1: CHECK THE INSULIN
STEP 2: ATTACH THE NEEDLE
STEP 3: PERFORM A SAFETY TEST
STEP 4: SELECT THE DOSE
STEP 5: INJECT THE DOSE
STEP 6: REMOVE AND DISCARD NEEDLE
It is important for you to go over these instructions carefully before using your SoloSTAR® pen.
SUPER: STEP 1: CHECK THE INSULIN
Laura: Step 1: Check the insulin.
SUPER: STEP 1: CHECK THE INSULIN
An unopened SoloSTAR® Pen should be refrigerated until first use. Do not store an opened SoloSTAR® pen in a refrigerator.
Laura: If your SoloSTAR® pen is in cool storage, take it out 1 to 2 hours before you inject to allow it to warm up. Cold insulin may be painful to inject.
SUPER: STEP 1: CHECK THE INSULIN
Check the label on your SoloSTAR® pen
Laura: First, check the label on your SoloSTAR® pen to make sure you have the correct pen and insulin. The Lantus® SoloSTAR® pen is gray with a purple injection button.
Check the expiration date on the label of your pen. Do not use a SoloSTAR® pen after the expiration date.
SUPER: STEP 1: CHECK THE INSULIN
Do not use the pen if the insulin is cloudy, colored, or has particles.
Laura:Take off the pen cap. Check the appearance of the insulin. Lantus® is a clear insulin. Do not use the pen if the insulin is cloudy, colored, or has particles.
SUPER: STEP 2: ATTACH THE NEEDLE
Laura: Step 2: Attach the needle.
SUPER: STEP 2: ATTACH THE NEEDLE
Laura: Laura: Do not reuse needles. Always use a new sterile needle for each injection. This helps prevent contamination and potential needle blocks.
SUPER: STEP 2: ATTACH THE NEEDLE
Wipe the rubber seal.
Laura: Wipe the rubber seal with alcohol.
SUPER: STEP 2: ATTACH THE NEEDLE
Remove protective seal.
DO NOT reuse needles.
Laura: Remove the protective seal from a new needle.
SUPER: STEP 2: ATTACH THE NEEDLE
Keep the needle straight as you attach it.
Laura: Line up the needle with the pen, and keep it straight as you attach it. Depending on the needle type, it may be pushed on or screwed on. If the needle is not kept straight while you attach it, it can damage the rubber seal and cause leakage, or break the needle.
SUPER: STEP 3: PERFORM A SAFETY TEST
Laura: Step 3: Perform a safety test.
SUPER: STEP 3: PERFORM A SAFETY TEST
Laura: Always perform the safety test before each injection.
Performing the safety test ensures you get an accurate dose. It ensures that the pen and needle work properly, and it also removes air bubbles in the insulin.
SUPER: STEP 3: PERFORM A SAFETY TEST
Select a dose of 2 units.
Laura:Select a dose of 2 units by turning the dosage selector.
SUPER: STEP 3: PERFORM A SAFETY TEST
Take off the outer needle cap.
Laura:Take off the outer needle cap and keep it to remove the used needle after injection.
SUPER: STEP 3: PERFORM A SAFETY TEST
Take off the inner needle cap.
Laura: Take off the inner needle cap and discard it.
SUPER: STEP 3: PERFORM A SAFETY TEST
Hold the pen pointing up, and tap the insulin reservoir.
Laura: Hold the pen with the needle pointing upwards. Then tap the insulin reservoir so that any air bubbles rise up towards the needle.
SUPER: STEP 3: PERFORM A SAFETY TEST
Laura: Press the button all the way in.
SUPER: STEP 3: PERFORM A SAFETY TEST
Check if insulin comes out of the needle tip.
Laura: Check if insulin comes out of the needle tip. You may have to perform the safety test several times before insulin is seen. If no insulin comes out, check for air bubbles and repeat the safety test two more times to remove them.
SUPER: STEP 3: PERFORM A SAFETY TEST
If insulin still does not come out, even after changing the needle, do not use it.
Do NOT use a syringe to remove Lantus® from your SoloSTAR® pen.
Laura: If insulin still does not come out, the needle may be blocked. Change the needle and try again. If no insulin comes out after changing the needle, your SoloSTAR® pen may be damaged. Do not use it.
SUPER: STEP 4: SELECT THE DOSE
Laura: Step 4: Select the dose.
SUPER: STEP 4: SELECT THE DOSE
Laura: You can set the dose in steps of 1 unit, from a minimum of 1 unit to a maximum of 80 units. If you need a dose greater than 80 units, you should give it as two or more doses. Check that the dose window shows "0" following the safety test.
SUPER: STEP 4: SELECT THE DOSE
Select your required dose. Ask for help if you have problems handling the pen, for example, if you have problems with your eyesight.
Laura: Select your required dose. If you turn past your dose, you can turn the dial back down. Do not push the injection button while turning, as insulin will come out.
SUPER: STEP 4: SELECT THE DOSE
Laura: You cannot turn the dosage selector past the number of units left in the pen. Do not force the dosage selector to turn. If what remains is less than you need, either you can inject what is remaining in the pen and complete your dose with a new SoloSTAR® pen, or you can use a new SoloSTAR® pen for your full dose.
SUPER: STEP 5: INJECT THE DOSE
Laura: Step 5: Inject the dose.
Laura: Use the injection method as instructed by your healthcare professional.
SUPER: The injection site should be changed each time you inject. DO NOT use the exact spot for each injection.
1 Stomach 2 Upper Arms 3 Thighs
Laura: You can inject Lantus® in three areas of your body. Anywhere in your stomach area, except for a two-inch radius around your navel. In the fatty tissue on the outer back area of your upper arm. Or in your thighs. Remember, the injection site should be changed each time you inject. Clean the injection site with an alcohol swab. Then insert the needle into the skin.
SUPER: STEP 5: INJECT THE DOSE
Deliver the dose by pressing the injection button in all the way.
Laura: Deliver the dose by pressing the injection button in all the way. The number in the dose window will return to "0" as you inject.
SUPER: STEP 5: INJECT THE DOSE
Keep the button pressed, and slowly count to 10.
Laura: Keep the button pressed all the way in. Slowly count to 10 before you withdraw the needle from the skin. This ensures that the full dose will be delivered.
SUPER: STEP 6: REMOVE AND DISCARD NEEDLE
Laura: Step 6: Remove and discard the needle.
Laura: Always remove the needle after each dose and store your SoloSTAR® pen without a needle attached. This helps prevent contamination and/or infection, as well as entry of air into the insulin reservoir and leakage of insulin, which can cause inaccurate dosing.
SUPER: STEP 6: REMOVE AND DISCARD NEEDLE
Put the outer needle cap on, and use it to unscrew the needle.
Laura: Always remove the needle after each dose and store your SoloSTAR® pen without a needle attached. This helps prevent contamination and/or infection, as well as entry of air into the insulin reservoir and leakage of insulin, which can cause inaccurate dosing.
Now I’m going to show you how to safely dispose of the needle.
SUPER: STEP 6: REMOVE AND DISCARD NEEDLE
Used needles should be placed in Sharps containers.
Laura: Used needles should be placed in sharps containers (such as red biohazard containers), hard plastic containers (such as detergent bottles), or metal containers (such as an empty coffee can). Such containers should be sealed and disposed of properly. If you are giving an injection to a third person, you should remove the needle in an approved manner to avoid needle-stick injuries.
SUPER: STEP 6: REMOVE AND DISCARD NEEDLE
Always put the cap back on before storing the pen.
Laura: Always put the pen cap back on the pen before storing the pen until your next injection.
SUPER: STORING YOUR SoloSTAR® PEN
Laura: Storing your pen.
Laura:Please review the leaflet that came with your Lantus® prescription for complete instructions on how to use and store the Lantus® SoloSTAR® pen. If your SoloSTAR® pen is in cool storage, take it out 1 to 2 hours before you use it to allow it to warm up. Cold insulin may be painful to inject. Keep the SoloSTAR® pen out of the reach and sight of children.
SUPER: STORING YOUR SoloSTAR® PEN
Keep an unopened SoloSTAR® pen in cool storage until first use. Between 36ºF and 46ºF (2ºC - 8ºC)
Do not:
Laura: Keep your unopened SoloSTAR® pen in cool storage until first use. Cool storage is between 36 and 46 degrees Fahrenheit, or 2 and 8 degrees Celsius. Do not allow it to freeze. Do not put it next to the freezer compartment of your refrigerator, or next to a freezer pack.
SUPER: STORING YOUR SoloSTAR® PEN
28 DAYS Do not store an opened SoloSTAR® pen in a refrigerator.
Discard an opened SoloSTAR® pen after 28 days.
Once a SoloSTAR® pen is in use, it must not be stored in a refrigerator.
Laura: Once you take your SoloSTAR® pen out of cool storage, for use or as a spare, you can use it for up to 28 days. During this time it can be safely kept at room temperature, up to 86 degrees Fahrenheit or 30 degrees Celsius. Do not use it after this time.
SUPER: STORING YOUR SoloSTAR® PEN
Laura: Do not use a SoloSTAR® pen after the expiration date printed on the label of the pen or the label on the carton. Protect your SoloSTAR® pen from light. Discard your used SoloSTAR® pen as required by your local authorities.
SUPER: CARING FOR YOUR SoloSTAR® PEN
Laura: Caring for your SoloSTAR® pen. Protect your SoloSTAR® pen from dust and dirt. You can clean the outside of your SoloSTAR® pen by wiping it with a damp cloth. Do not soak, wash, or lubricate the pen as this may damage it. Your SoloSTAR® pen is designed to work accurately and safely. It should be handled with care. Avoid situations where the SoloSTAR® pen might be damaged. If you are concerned that your SoloSTAR® pen may be damaged, use a new one. If you have any further questions about the Lantus® SoloSTAR® pen or managing your diabetes, be sure to speak to your doctor, nurse, or pharmacist.
Thanks for joining me on this walk through how to use the Lantus® SoloSTAR® pen. I hope that you found these instructions helpful. Now, I will present the full Important Safety Information for Lantus®.
Laura: What is Lantus® (insulin glargine injection) 100 Units/mL?
Prescription LANTUS is a long-acting man-made-insulin used to control high blood sugar in adults and children with diabetes mellitus.
Important Safety Information for Lantus (insulin glargine injection) 100 Units/mL
Do not take Lantus during episodes of low blood sugar or if you are allergic to insulin or any of the inactive ingredients in Lantus.
Do not share needles, insulin pens, or syringes with others. Do NOT reuse needles.
Before starting Lantus, tell your doctor about all your medical conditions, including if you have liver or kidney problems, if you are pregnant or planning to become pregnant or if you are breast-feeding or planning to breast-feed.
Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting lipodystrophy (pitted or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
Heart failure can occur if you are taking insulin together with certain medicines called TZDs (thiazolidinediones), even if you have never had heart failure or other heart problems. If you already have heart failure, it may get worse while you take TZDs with Lantus. Your treatment with TZDs and Lantus may need to be changed or stopped by your doctor if you have new or worsening heart failure. Tell your doctor if you have any new or worsening symptoms of heart failure, including:
Tell your doctor about all the medications you take, including OTC medicines, vitamins, and supplements, including herbal supplements.
Lantus should be taken once a day at the same time every day. Test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare provider. Any change of insulin should be made cautiously and only under medical supervision.
Do NOT dilute or mix Lantus with any other insulin or solution. It will not work as intended and you may lose blood sugar control, which could be serious. Lantus must only be used if the solution is clear and colorless with no particles visible. Always make sure you have the correct insulin before each injection.
While using Lantus, do not drive or operate heavy machinery until you know how Lantus affects you. You should not drink alcohol or use other medicines that contain alcohol.
The most common side effect of insulin, including Lantus, is low blood sugar (hypoglycemia), which may be serious and life threatening. It may cause harm to your heart or brain. Symptoms of serious low blood sugar may include shaking, sweating, fast heartbeat, and blurred vision.
Lantus may cause serious side effects that can lead to death, such as severe allergic reactions. Get medical help right away if you have:
Other possible side effects may include swelling, weight gain, low potassium levels, injection site reactions, including changes in fat tissue at the injection site, and allergic reactions.
Important Safety Information for Lantus (insulin glargine injection) SoloStar
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. Please talk to your healthcare provider about proper injection technique and follow instructions in the Instruction Leaflet that accompanies the pen.
SUPER: Please see full Important Safety Information for Lantus® (insulin glargine injection) 100 Units/mL at the end of this video.
Please see full Prescribing Information at the link on this website.
MAT-US-2006643-v3.0-11/2022
GETTING THE DOSAGE THAT’S RIGHT FOR YOU
Whether you’re new to using insulin or already on insulin, expect your dose to be adjusted by your doctor over time. This is called titration.
YOUR DOCTOR WILL BASE YOUR DOSE ON:
- Your blood sugar testing results
- Your blood sugar goal number
- Your needs
HOW TO INJECT LANTUS WITH A VIAL AND SYRINGE
If your doctor told you to take Lantus using a vial and syringe, this presentation can help walk you through it, step by step.
1. Before You Get Started:
- Wash your hands
- Make sure the insulin is clear and colorless. Do not use it if it is cloudy or if you see particles; throw it away
- Do not mix or dilute Lantus® with any other insulin or solution. It will not work as intended, and you may lose blood sugar control
- Do not share needles, insulin pens, or syringes with others
- Do NOT reuse needles. Always use a new syringe
2. Prepare the Dose:
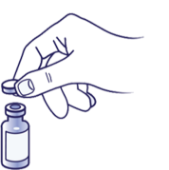
Remove the cap:
If you are using a new vial, remove the protective cap. Do not remove the stopper
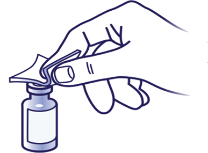
Sterilize the top:
Wipe the top of the vial with an alcohol swab.
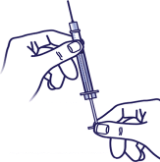
Inject air into the vial:
Draw air into the syringe that is equal to your insulin dose.
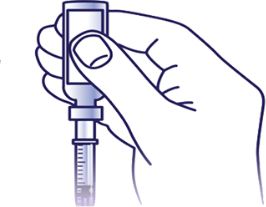
Put the needle:
Put the needle through the rubber top of the vial and push the plunger to inject the air into the vial.
Draw up the dose:
Leave the syringe in the vial and turn both upside down. Hold the syringe and vial firmly in one hand. Make sure the tip of the needle is in the insulin. With your free hand, pull the plunger to withdraw the correct dose into the syringe.
3. Remove Air Bubbles:

Check for Bubbles:
Before you take the needle out of the vial, check the syringe for air bubbles.
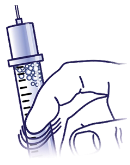
Tap to Release:
If bubbles are in the medicine, hold the syringe straight up and tap the side of the syringe until the bubbles float to the top.
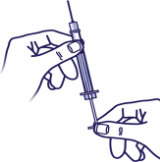
Eject the Air:
Push the bubbles out with the plunger and draw insulin back in until you have the correct dose.
Remove the Needle:
Remove the needle from the vial. Do not let the needle touch anything. You’re now ready to inject.
4. Choose an Injection Area:
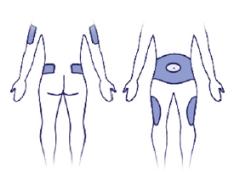
Pick your Spot:
Decide on injection area: either upper arm, thigh, or abdomen. Injection sites within an injection area must be different from one injection to the next.

Clean your Skin:
Using rubbing alcohol to clean the skin where you are going to inject. Alcohol can sometimes sting if it’s not completely dry when you inject, so wait a few seconds for it to evaporate or pat the area dry with a sterile cotton ball.

Pinch a Fold of Skin:
Pinch the skin and hold it. Insert the needle the way your healthcare professional showed you.
5. Complete Injection:
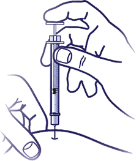
Administer Insulin:
Slowly push in the plunger of the syringe all the way, making sure you have injected all the insulin. Leave the needle in the skin for 10 seconds.

Apply Light Pressure:
Pull the needle straight out and gently press on the spot where you injected yourself for several seconds. Don’t rub the area.

Discard Materials Safely:
Follow your healthcare professional’s instructions for throwing away the needle and syringe.
For instructions on how to inject Lantus, please talk to your treating healthcare provider and refer to the instruction leaflet that came with your vial.
BE SYRINGE SAVVY
To make sure you get the right dose of insulin, always use a U-100 insulin syringe. (Your pharmacist can help you make sure you have the right syringe.) If you have trouble seeing the volume lines on a syringe, ask your healthcare team or pharmacist for a magnifying device that can help.
STORAGE INSTRUCTIONS
-
Store unused Lantus vials in the refrigerator between 36˚F to 46˚F (2˚C to 8˚C)
- Store in-use (opened) Lantus vials in a refrigerator or at room temperature below 86˚F (30˚C)
- Do not freeze Lantus
- Keep Lantus out of direct heat and light
- If a vial has been frozen or overheated, throw it away
- The Lantus vials you are using should be thrown away after 28 days, even if it still has insulin left in it
LANTUS® SIDE EFFECTS
Common and serious side effects: What to watch for
The most common side effect of insulin, including Lantus, is low blood sugar (hypoglycemia), which may be serious. Some people may experience symptoms such as shaking, sweating, fast heartbeat, and blurred vision.
Ask your doctor about the symptoms and signs of hypoglycemia, how to track your blood sugar, and what to do if you suffer a hypoglycemic event.
Other possible side effects
Lantus may cause serious side effects.
Get medical help right away if you have:
- A rash over your whole body
- Trouble breathing
- A fast heartbeat
- Sweating
- Swelling of your face, throat, or tongue
- Shortness of breath
- Extreme drowsiness, confusion, or dizziness
These are not all the possible side effects of Lantus. Speak with your doctor about possible side effects.
For more detailed information, see the Important Safety Information for Lantus and Full Prescribing Information.
Not actual patients.
QUESTIONS? SEE OUR FAQS
If you’re like lots of folks, you may have questions.
We’ll give you the answers to some of the most common questions asked about diabetes - and Lantus.
- A rash over your whole body
- Trouble breathing
- A fast heartbeat
- Sweating
- Swelling of your face, tongue, or throat
- Shortness of breath
- Extreme drowsiness, dizziness, or confusion
- Commercially insured patients pay no more than $35* per 30-day supply. *See Terms and Eligibility Restrictions
- What if I don’t have insurance or prefer to pay cash outside my prescription drug benefit? Can I still save on Lantus? Yes. If you’re uninsured or insured and prefer to pay cash outside of your prescription drug benefit, you will pay $35* per 30-day supply of any one or combination of Sanofi Insulins through the Insulins Valyou Savings Program*. *See Terms and Eligibility Restrictions
Lantus is an injection administered under the skin. This is called a “subcutaneous” injection. There are two ways you can inject Lantus: with the Lantus SoloStar® pen, or with a vial and syringe.
Weight gain can occur with any insulin therapy, including Lantus. Following a healthy diet and exercise program can help with weight control. Try some of our healthy recipes to help you stay on track.
The most common side effect of insulin, including Lantus®, is low blood sugar (hypoglycemia), which may be serious and life threatening. It may cause harm to your heart and brain. Symptoms of serious low blood sugar may include shaking, sweating, fast heartbeat, and blurred vision.
Lantus may cause serious side effects that can lead to death, such as severe allergic reactions. Get medical help right away if you have:
No. Do not mix Lantus with any other insulin or solution. It will not work as intended, and you may lose blood sugar control, which could be serious.
Your doctor will guide you on any adjustments that need to be made. So, if you’re also taking mealtime insulin, you should talk to your doctor about any changes you may need to make to your mealtime insulin dose.
No. Only Lantus is Lantus. Proven to lower A1C and help control blood sugar for a full 24 hours, it’s a choice for physicians and millions of patients worldwide. While there are now similar medicines (called biosimilars) available, Lantus is the most prescribed long-acting insulin1. If you are eligible and have a private or commercial insurance, you will pay no more than $35 for a 30 day-supply. If you have Medicare Part D insurance, it’s good to know that 7 out of 10 patients receive prescription coverage of Lantus at the lowest branded copay. Lantus also participates in our Insulin Valyou Savings Plan where patients without any insurance or commercially insured patients paying cash pay $35 for a monthly supply of one or a combination of Sanofi Insulins.
1Based on TRx data from IMS Health, NPATM monthly database, time period from May 2003 to July 2025.
Lantus and Toujeo are both once-daily, long-acting insulins. Use the chart to see how they compare.
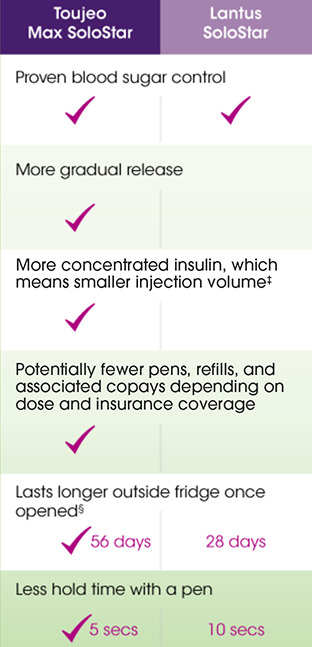
Interested?
Talk with your doctor about how Toujeo compares with Lantus, or visit toujeo.com
Toujeo Max SoloStar is recommended for appropriate patients who require at least 20 units of basal insulin per day.
†One unit of Toujeo has the smallest injection volume compared to 1 unit of any other long-acting insulin.
§At room temperature below 86°F (30°C). ‡Toujeo SoloStar study performed in a laboratory environment; doses were not delivered into tissue.
Please see Full Prescribing Information
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (8:00 am-8:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is only valid for those who are uninsured or those who are insured by a prescription plan but are not using such insurance and will be paying the full retail price for the medication. Void where prohibited by law. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. You may not submit claims for reimbursement to any third-party payor, including any government healthcare plan (e.g., Medicare, Medicaid, DOD, VA, TRICARE) or similar federal or state programs for Sanofi Insulin prescriptions when using this Program. You may not seek to have your out-of-pocket costs or the full retail price of the Sanofi Insulin count toward your deductible, true-out-of-pocket (TrOOP), maximum out-of-pocket (MOOP), or any other out-of-pocket caps associated with any insurance coverage. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (8:00 am-8:00 pm EST, Monday-Friday).
Developed by the makers of Lantus, SOLIQUA 100/33 combines Lantus with lixisenatide, a non-insulin diabetes medicine.
Find out more at soliqua100-33.com
Please see Full Indication, Limitations of Use and Important Safety Information for SOLIQUA 100/33
Please see Full Prescribing Information
Yes. If you’re uninsured or insured and prefer to pay cash outside of your prescription drug benefit, you will pay $35* per 30-day supply of any one or combination of Sanofi Insulins through the Insulins Valyou Savings Program*.

GOT QUESTIONS?
See our FAQs. We’ll give you the answers to some of the most common questions asked about diabetes—and Lantus.

SAVINGS OFFERS
Whether you have commercial insurance or not, or prefer to pay cash outside of your prescription drug benefit, we have an offer for you.
*See Terms and Eligibility Restrictions

READY TO USE LANTUS?
Check out the “How to Use” video, learn about adjusting your dose, and more.
What is Lantus® (insulin glargine injection) 100 Units/mL?
Prescription LANTUS is a long-acting man-made-insulin used to control high blood sugar in adults and children with diabetes mellitus.
- LANTUS is not for use to treat diabetic ketoacidosis
Important Safety Information
Important Safety Information
Do not take Lantus during episodes of low blood sugar or if you are allergic to insulin or any of the inactive ingredients in Lantus.
Do not share needles, insulin pens, or syringes with others. Do NOT reuse needles.
Before starting Lantus, tell your doctor about all your medical conditions, including if you have liver or kidney problems, if you are pregnant or planning to become pregnant or if you are breast-feeding or planning to breast-feed.
Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting lipodystrophy (pitted or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
Heart failure can occur if you are taking insulin together with certain medicines called TZDs (thiazolidinediones), even if you have never had heart failure or other heart problems. If you already have heart failure, it may get worse while you take TZDs with Lantus. Your treatment with TZDs and Lantus may need to be changed or stopped by your doctor if you have new or worsening heart failure. Tell your doctor if you have any new or worsening symptoms of heart failure, including:
- Shortness of breath
- Swelling of your ankles or feet
- Sudden weight gain
Tell your doctor about all the medications you take, including OTC medicines, vitamins, and supplements, including herbal supplements.
Lantus should be taken once a day at the same time every day. Test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare provider. Any change of insulin should be made cautiously and only under medical supervision.
Do NOT dilute or mix Lantus with any other insulin or solution. It will not work as intended and you may lose blood sugar control, which could be serious. Lantus must only be used if the solution is clear and colorless with no particles visible. Always make sure you have the correct insulin before each injection.
While using Lantus, do not drive or operate heavy machinery until you know how Lantus affects you. You should not drink alcohol or use other medicines that contain alcohol.
The most common side effect of insulin, including Lantus, is low blood sugar (hypoglycemia), which may be serious and life threatening. It may cause harm to your heart or brain. Symptoms of serious low blood sugar may include shaking, sweating, fast heartbeat, and blurred vision.
Lantus may cause serious side effects that can lead to death, such as severe allergic reactions. Get medical help right away if you have:
- A rash over your whole body
- Trouble breathing
- A fast heartbeat
- Sweating
- Swelling of your face, tongue, or throat
- Shortness of breath
- Extreme drowsiness, dizziness, or confusion
Other possible side effects may include swelling, weight gain, low potassium levels, injection site reactions, including changes in fat tissue at the injection site, and allergic reactions.
Important Safety Information for Lantus (insulin glargine injection) SoloStar
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. Please talk to your healthcare provider about proper injection technique and follow instructions in the Instruction Leaflet that accompanies the pen.
Click here for Full Prescribing Information for Lantus.
What is Toujeo U-300 (insulin glargine) injection 300 Units/mL?
Prescription Toujeo is a long-acting man-made insulin used to control high blood sugar in adults and children who are 6 years of age and older with diabetes mellitus.
- Toujeo is not for the treatment of diabetic ketoacidosis
- It is not known if Toujeo is safe and effective in children under 6 years of age
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Do not use Toujeo if you have low blood sugar or if you are allergic to insulin or any of the ingredients in Toujeo.
Do not share your pen(s) with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Before starting Toujeo, tell your doctor about all your medical conditions, including if you have liver or kidney problems, if you are pregnant or planning to become pregnant, or if you are breastfeeding or planning to breastfeed.
Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting pitted or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis ) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred, or damaged.
Heart failure can occur if you are taking insulin together with pills called TZDs (thiazolidinediones), even if you have never had heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with Toujeo. Your treatment with TZDs and Toujeo may need to be changed or stopped by your doctor if you have new or worsening heart failure. Tell your doctor if you have any new or worsening symptoms including:
- Shortness of breath
- Sudden weight gain
- Swelling of your ankles or feet
Tell your doctor about all the medications you take, including OTC medicines, vitamins, and supplements, and herbal supplements.
Toujeo should be taken at the same time once a day. Test your blood sugar levels daily while using any insulin. Do not change your dose or type of insulin without talking to your doctor. Verify you have the correct insulin before each injection. Do NOT use a syringe to remove Toujeo from your pen. Your dose for Toujeo may be different from other insulins you have taken. Any change of insulin should be made cautiously and only under medical supervision.
Do NOT dilute or mix Toujeo with any other insulin or solution. It will not work as intended and you may lose blood sugar control, which could be serious. Use Toujeo only if the solution is clear and colorless with no particles visible.
While using Toujeo, do not drive or operate heavy machinery until you know how Toujeo affects you. Don’t drink alcohol or use other medicines that contain alcohol.
The most common side effect of Toujeo is low blood sugar (hypoglycemia), which may be serious and life-threatening. Severe hypoglycemia may cause harm to your heart or brain. Symptoms of serious low blood sugar may include shaking, sweating, fast heartbeat, and blurred vision. The long-acting effect of Toujeo may delay recovery from low blood sugar compared to shorter-acting insulins.
Toujeo may cause severe allergic reactions that can lead to death. Get medical help right away if you have:
- A rash over your whole body
- Shortness of breath
- Swelling of your face, tongue, or throat
- Extreme drowsiness, dizziness, or confusion
- Trouble breathing
- Fast heartbeat
- Sweating
Toujeo may have additional side effects including swelling, weight gain, low potassium, and injection site reactions which may include change in fat tissue, skin thickening, redness, swelling, and itching.
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. It is important to perform a safety test when using a new pen for the first time. Talk to your doctor about proper injection technique and follow instructions in the Instruction Leaflet that comes with your Toujeo SoloStar or Toujeo Max SoloStar pen.
Click here for Full Prescribing Information for Toujeo.
SOLIQUA 100/33 is an injectable prescription medicine that contains 2 diabetes medicines, insulin glargine and lixisenatide, that is used along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes.
- It is not recommended for people who also take lixisenatide or other medicines called glucagonlike peptide 1 (GLP-1) receptor agonists.
- It is not for use in people with diabetic ketoacidosis.
- It has not been studied in people who also take a short-acting (prandial) insulin.
- It is not known if SOLIQUA 100/33 is safe and effective in children.
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
What is the most important information I should know about SOLIQUA 100/33?
SOLIQUA 100/33 can cause serious side effects, including inflammation of the pancreas(pancreatitis)
Stop using SOLIQUA 100/33 and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Who should not use SOLIQUA 100/33?
Do not use SOLIQUA 100/33 if you:
- are having an episode of low blood sugar (hypoglycemia)
- have had a serious allergic reaction to insulin glargine, lixisenatide, or any of the ingredients in SOLIQUA 100/33. See “What are the possible side effects of SOLIQUA 100/33?” for symptoms of a serious allergic reaction.
Before using SOLIQUA 100/33, tell your healthcare provider about all your medical conditions, including if you:
- have or have had problems with your pancreas.
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take thiazolidinediones (TZDs).
- have severe problems with your stomach, such as slowed emptying of your stomach or problems digesting food.
- are taking certain medicines called glucagon-like peptide 1 receptor agonists (GLP-1 receptor agonists).
- are scheduled to have surgery or other procedures that use general anesthesia or deep sleepiness (deep sedation)
- are pregnant or breastfeeding or plan to become pregnant or to breastfeed. It is not known if SOLIQUA 100/33 will harm your unborn baby or pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including all prescription and over-thecounter medicines, vitamins, and herbal supplements. SOLIQUA 100/33 may affect the way some medicines work.
Before using SOLIQUA 100/33, talk to your healthcare provider about low blood sugar and how to manage it.
Especially tell your healthcare provider if you take:
- antibiotics or the pain reliever, acetaminophen. Take these medicines at least 1 hour before using SOLIQUA 100/33. If you must take these medicines, take them with a meal or a snack. You should not take these medicines at the same time that you take SOLIQUA 100/33.
- birth control pills that are taken by mouth (oral contraceptives). SOLIQUA 100/33 may lower the amount of the medicine in your blood from your birth control pills and they may not work as well to prevent pregnancy. Take your birth control pill at least 1 hour before your injection of SOLIQUA 100/33 or at least 11 hours after your SOLIQUA 100/33 injection.
How should I use SOLIQUA 100/33?
-
Do not change your dose without first talking to your healthcare provider.
- Your healthcare provider should show you how to use SOLIQUA 100/33 before you use it for the first time.
- Check the pen label each time you inject to make sure you are using the correct medicine.
- Do not take more than 60 units of SOLIQUA 100/33 each day. Do not take SOLIQUA 100/33 with other GLP-1 receptor agonists.
- Only use SOLIQUA 100/33 that is clear and colorless to almost colorless. If you see small particles, return it to your pharmacy for replacement.
- Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting pitted or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
- Do not remove SOLIQUA 100/33 from the pen with a syringe.
- Do not re-use or share needles with other people. You may give other people a serious infection, or get a serious infection from them.
- If you take too much SOLIQUA 100/33, call your healthcare provider or the Poison Help line at 1800-222-1222 or go to the nearest hospital emergency room right away.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check.
What should I avoid while taking SOLIQUA 100/33?
While taking SOLIQUA 100/33 do not:
- Drive or operate heavy machinery, until you know how SOLIQUA 100/33 affects you.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of SOLIQUA 100/33?
SOLIQUA 100/33 can cause serious side effects including:
- See "What is the most important information I should know about SOLIQUA 100/33?"
- Severe allergic reactions. Stop taking SOLIQUA 100/33 and get medical help right away if you have any symptoms of a severe allergic reaction including
- swelling of your face, lips, tongue or throat
- fainting or feeling dizzy
- problems breathing or swallowing
- very rapid heartbeat
- severe rash or itching
- swelling of your face, lips, tongue or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar is higher if you take another medicine that can cause low blood sugar.
Signs and symptoms of low blood sugar include:- dizziness or lightheadedness
- blurred vision
- anxiety, irritability or mood changes
- sweating
- slurred speech
- hunger
- confusion or drowsiness
- shakiness
- weakness
- headache
- fast heartbeat
- feeling jittery
- Dizziness or light-headedness
- fast heartbeat
- sweating
- anxiety, irritability or mood changes
- confusion or drowsiness
- hunger
- headache
- weakness
- blurred vision
- feeling jittery
- slurred speech
- shakiness
- Dehydration leading to Kidney problems. Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration. Tell your healthcare provider right away if you have nausea, vomiting, or diarrhea that does not go away.
- Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use SOLIQUA 100/33. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called TZDs with SOLIQUA 100/33 may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with SOLIQUA 100/33. Your healthcare provider should monitor you closely while you are taking TZDs with SOLIQUA 100/33. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, swelling of your ankles or feet, or sudden weight gain. Treatment with TZDs and SOLIQUA 100/33 may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
- Gallbladder problems. Gallbladder problems have happened in some people who take SOLIQUA 100/33. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in your upper stomach (abdomen)
- yellowing of skin or eyes (jaundice)
- fever
- clay-colored stools
- pain in your upper stomach (abdomen)
- fever
- yellowing of skin or eyes (jaundice)
- clay-colored stools
- Food or liquid getting into the lungs during surgery or other procedures that use general anesthesia or deep sleepiness (deep sedation). SOLIQUA 100/33 may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking SOLIQUA 100/33 before you are scheduled to have a surgery or other procedures.
The most common side effects of SOLIQUA 100/33 include:
|
|
|
|
|
|
|
|
|
|
|
|
Talk to your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of SOLIQUA 100/33. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 1-800-FDA-1088.
Click here for Full Prescribing Information for SOLIQUA 100/33.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. For the duration of the program, eligible commercially insured patients pay no more than $35 per 30-day supply, up to 10 packs per fill; Offer valid for one fill every 30 days. Savings may vary depending on patients’ out-of-pocket costs. The Sanofi Insulins Co-pay Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (9:00 am-7:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is only valid for those who are uninsured or those who are insured by a prescription plan but are not using such insurance and will be paying the full retail price for the medication. Void where prohibited by law. For the duration of the program, eligible patients will pay $35 per 30-day supply. To pay $35 per 30-day supply, you must fill all your Sanofi Insulin prescriptions at the same time, together each month. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. You may not submit claims for reimbursement to any third-party payor, including any government healthcare plan (e.g., Medicare, Medicaid, DOD, VA, TRICARE) or similar federal or state programs for Sanofi Insulin prescriptions when using this Program. You may not seek to have your out-of-pocket costs or the full retail price of the Sanofi Insulin count toward your deductible, true-out-of-pocket (TrOOP), maximum out-of-pocket (MOOP), or any other out-of-pocket caps associated with any insurance coverage. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (9:00 am-7:00 pm EST, Monday-Friday).
References:
1. Lantus Prescribing Information.
2. Toujeo Prescribing Information.
If you are a patient experiencing problems with a Sanofi US product, please contact Sanofi US at 1-800-633-16101-800-633-1610.
The health information contained herein is provided for general education purposes only. Your healthcare professional is the single best source of information regarding your health. Please consult your healthcare professional if you have any questions about your health or treatment.
Important Safety Information
Do not take Lantus during episodes of low blood sugar or if you are allergic to insulin or any of the inactive ingredients in Lantus.
Do not share needles, insulin pens, or syringes with others. Do NOT reuse needles.
Before starting Lantus, tell your doctor about all your medical conditions, including if you have liver or kidney problems, if you are pregnant or planning to become pregnant or if you are breast-feeding or planning to breast-feed.
Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting lipodystrophy (pitted or thickened skin) and localized cutaneous amyloidosis (skin with lumps) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
Heart failure can occur if you are taking insulin together with certain medicines called TZDs (thiazolidinediones), even if you have never had heart failure or other heart problems. If you already have heart failure, it may get worse while you take TZDs with Lantus. Your treatment with TZDs and Lantus may need to be changed or stopped by your doctor if you have new or worsening heart failure. Tell your doctor if you have any new or worsening symptoms of heart failure, including:
- Shortness of breath
- Swelling of your ankles or feet
- Sudden weight gain
Tell your doctor about all the medications you take, including OTC medicines, vitamins, and supplements, including herbal supplements.
Lantus should be taken once a day at the same time every day. Test your blood sugar levels while using insulin, such as Lantus. Do not make any changes to your dose or type of insulin without talking to your healthcare provider. Any change of insulin should be made cautiously and only under medical supervision.
Do NOT dilute or mix Lantus with any other insulin or solution. It will not work as intended and you may lose blood sugar control, which could be serious. Lantus must only be used if the solution is clear and colorless with no particles visible. Always make sure you have the correct insulin before each injection.
While using Lantus, do not drive or operate heavy machinery until you know how Lantus affects you. You should not drink alcohol or use other medicines that contain alcohol.
The most common side effect of insulin, including Lantus, is low blood sugar (hypoglycemia), which may be serious and life threatening. It may cause harm to your heart or brain. Symptoms of serious low blood sugar may include shaking, sweating, fast heartbeat, and blurred vision.
Lantus may cause serious side effects that can lead to death, such as severe allergic reactions. Get medical help right away if you have:
- A rash over your whole body
- Trouble breathing
- A fast heartbeat
- Sweating
- Swelling of your face, tongue, or throat
- Shortness of breath
- Extreme drowsiness, dizziness, or confusion
Other possible side effects may include swelling, weight gain, low potassium levels, injection site reactions, including changes in fat tissue at the injection site, and allergic reactions.
Important Safety Information for Lantus (insulin glargine injection) SoloStar
Lantus SoloSTAR is a disposable single-patient-use prefilled insulin pen. Please talk to your healthcare provider about proper injection technique and follow instructions in the Instruction Leaflet that accompanies the pen.
Click here for Full Prescribing Information for Lantus.
What is Toujeo U-300 (insulin glargine) injection 300 Units/mL?
Prescription Toujeo is a long-acting man-made insulin used to control high blood sugar in adults and children who are 6 years of age and older with diabetes mellitus.
- Toujeo is not for the treatment of diabetic ketoacidosis
- It is not known if Toujeo is safe and effective in children under 6 years of age
Important Safety Information for Toujeo U-300 (insulin glargine) injection
Do not use Toujeo if you have low blood sugar or if you are allergic to insulin or any of the ingredients in Toujeo.
Do not share your pen(s) with other people, even if the needle has been changed. You may give other people a serious infection or get a serious infection from them.
Before starting Toujeo, tell your doctor about all your medical conditions, including if you have liver or kidney problems, if you are pregnant or planning to become pregnant, or if you are breastfeeding or planning to breastfeed.
Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting pitted or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis ) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred, or damaged.
Heart failure can occur if you are taking insulin together with pills called TZDs (thiazolidinediones), even if you have never had heart failure or other heart problems. If you have heart failure, it may get worse while you take TZDs with Toujeo. Your treatment with TZDs and Toujeo may need to be changed or stopped by your doctor if you have new or worsening heart failure. Tell your doctor if you have any new or worsening symptoms including:
- Shortness of breath
- Sudden weight gain
- Swelling of your ankles or feet
Tell your doctor about all the medications you take, including OTC medicines, vitamins, and supplements, and herbal supplements.
Toujeo should be taken at the same time once a day. Test your blood sugar levels daily while using any insulin. Do not change your dose or type of insulin without talking to your doctor. Verify you have the correct insulin before each injection. Do NOT use a syringe to remove Toujeo from your pen. Your dose for Toujeo may be different from other insulins you have taken. Any change of insulin should be made cautiously and only under medical supervision.
Do NOT dilute or mix Toujeo with any other insulin or solution. It will not work as intended and you may lose blood sugar control, which could be serious. Use Toujeo only if the solution is clear and colorless with no particles visible.
While using Toujeo, do not drive or operate heavy machinery until you know how Toujeo affects you. Don’t drink alcohol or use other medicines that contain alcohol.
The most common side effect of Toujeo is low blood sugar (hypoglycemia), which may be serious and life-threatening. Severe hypoglycemia may cause harm to your heart or brain. Symptoms of serious low blood sugar may include shaking, sweating, fast heartbeat, and blurred vision. The long-acting effect of Toujeo may delay recovery from low blood sugar compared to shorter-acting insulins.
Toujeo may cause severe allergic reactions that can lead to death. Get medical help right away if you have:
- A rash over your whole body
- Shortness of breath
- Swelling of your face, tongue, or throat
- Extreme drowsiness, dizziness, or confusion
- Trouble breathing
- Fast heartbeat
- Sweating
Toujeo may have additional side effects including swelling, weight gain, low potassium, and injection site reactions which may include change in fat tissue, skin thickening, redness, swelling, and itching.
Toujeo SoloStar and Toujeo Max SoloStar are single-patient-use prefilled insulin pens. It is important to perform a safety test when using a new pen for the first time. Talk to your doctor about proper injection technique and follow instructions in the Instruction Leaflet that comes with your Toujeo SoloStar or Toujeo Max SoloStar pen.
Click here for Full Prescribing Information for Toujeo.
SOLIQUA 100/33 is an injectable prescription medicine that contains 2 diabetes medicines, insulin glargine and lixisenatide, that is used along with diet and exercise to improve blood sugar (glucose) in adults with type 2 diabetes.
- It is not recommended for people who also take lixisenatide or other medicines called glucagonlike peptide 1 (GLP-1) receptor agonists.
- It is not for use in people with diabetic ketoacidosis.
- It has not been studied in people who also take a short-acting (prandial) insulin.
- It is not known if SOLIQUA 100/33 is safe and effective in children.
Important Safety Information for SOLIQUA 100/33 (insulin glargine and lixisenatide) injection 100 Units/mL and 33 mcg/mL
What is the most important information I should know about SOLIQUA 100/33?
SOLIQUA 100/33 can cause serious side effects, including inflammation of the pancreas(pancreatitis)
Stop using SOLIQUA 100/33 and call your healthcare provider right away if you have severe pain in your stomach area (abdomen) that will not go away, with or without vomiting. You may feel the pain from your abdomen to your back.
Who should not use SOLIQUA 100/33?
Do not use SOLIQUA 100/33 if you:
- are having an episode of low blood sugar (hypoglycemia)
- have had a serious allergic reaction to insulin glargine, lixisenatide, or any of the ingredients in SOLIQUA 100/33. See “What are the possible side effects of SOLIQUA 100/33?” for symptoms of a serious allergic reaction.
Before using SOLIQUA 100/33, tell your healthcare provider about all your medical conditions, including if you:
- have or have had problems with your pancreas.
- have heart failure or other heart problems. If you have heart failure, it may get worse while you take thiazolidinediones (TZDs).
- have severe problems with your stomach, such as slowed emptying of your stomach or problems digesting food.
- are taking certain medicines called glucagon-like peptide 1 receptor agonists (GLP-1 receptor agonists).
- are scheduled to have surgery or other procedures that use general anesthesia or deep sleepiness (deep sedation)
- are pregnant or breastfeeding or plan to become pregnant or to breastfeed. It is not known if SOLIQUA 100/33 will harm your unborn baby or pass into your breast milk.
Tell your healthcare provider about all the medicines you take, including all prescription and over-thecounter medicines, vitamins, and herbal supplements. SOLIQUA 100/33 may affect the way some medicines work.
Before using SOLIQUA 100/33, talk to your healthcare provider about low blood sugar and how to manage it.
Especially tell your healthcare provider if you take:
- antibiotics or the pain reliever, acetaminophen. Take these medicines at least 1 hour before using SOLIQUA 100/33. If you must take these medicines, take them with a meal or a snack. You should not take these medicines at the same time that you take SOLIQUA 100/33.
- birth control pills that are taken by mouth (oral contraceptives). SOLIQUA 100/33 may lower the amount of the medicine in your blood from your birth control pills and they may not work as well to prevent pregnancy. Take your birth control pill at least 1 hour before your injection of SOLIQUA 100/33 or at least 11 hours after your SOLIQUA 100/33 injection.
How should I use SOLIQUA 100/33?
-
Do not change your dose without first talking to your healthcare provider.
- Your healthcare provider should show you how to use SOLIQUA 100/33 before you use it for the first time.
- Check the pen label each time you inject to make sure you are using the correct medicine.
- Do not take more than 60 units of SOLIQUA 100/33 each day. Do not take SOLIQUA 100/33 with other GLP-1 receptor agonists.
- Only use SOLIQUA 100/33 that is clear and colorless to almost colorless. If you see small particles, return it to your pharmacy for replacement.
- Change (rotate) your injection sites within the area you chose with each dose to reduce your risk of getting pitted or thickened skin (lipodystrophy) and skin with lumps (localized cutaneous amyloidosis) at the injection sites. Do not use the same spot for each injection or inject where the skin is pitted, thickened, lumpy, tender, bruised, scaly, hard, scarred or damaged.
- Do not remove SOLIQUA 100/33 from the pen with a syringe.
- Do not re-use or share needles with other people. You may give other people a serious infection, or get a serious infection from them.
- If you take too much SOLIQUA 100/33, call your healthcare provider or the Poison Help line at 1800-222-1222 or go to the nearest hospital emergency room right away.
- Check your blood sugar levels. Ask your healthcare provider what your blood sugar should be and when you should check.
What should I avoid while taking SOLIQUA 100/33?
While taking SOLIQUA 100/33 do not:
- Drive or operate heavy machinery, until you know how SOLIQUA 100/33 affects you.
- Drink alcohol or use prescription or over-the-counter medicines that contain alcohol.
What are the possible side effects of SOLIQUA 100/33?
SOLIQUA 100/33 can cause serious side effects including:
- See "What is the most important information I should know about SOLIQUA 100/33?"
- Severe allergic reactions. Stop taking SOLIQUA 100/33 and get medical help right away if you have any symptoms of a severe allergic reaction including
- swelling of your face, lips, tongue or throat
- fainting or feeling dizzy
- problems breathing or swallowing
- very rapid heartbeat
- severe rash or itching
- swelling of your face, lips, tongue or throat
- problems breathing or swallowing
- severe rash or itching
- fainting or feeling dizzy
- very rapid heartbeat
- Low blood sugar (hypoglycemia). Your risk for getting low blood sugar is higher if you take another medicine that can cause low blood sugar.
Signs and symptoms of low blood sugar include:- dizziness or lightheadedness
- blurred vision
- anxiety, irritability or mood changes
- sweating
- slurred speech
- hunger
- confusion or drowsiness
- shakiness
- weakness
- headache
- fast heartbeat
- feeling jittery
- Dizziness or light-headedness
- fast heartbeat
- sweating
- anxiety, irritability or mood changes
- confusion or drowsiness
- hunger
- headache
- weakness
- blurred vision
- feeling jittery
- slurred speech
- shakiness
- Dehydration leading to Kidney problems. Diarrhea, nausea, and vomiting may cause a loss of fluids (dehydration) which may cause kidney problems. It is important for you to drink fluids to help reduce your chance of dehydration. Tell your healthcare provider right away if you have nausea, vomiting, or diarrhea that does not go away.
- Severe stomach problems. Stomach problems, sometimes severe, have been reported in people who use SOLIQUA 100/33. Tell your healthcare provider if you have stomach problems that are severe or will not go away.
- Low potassium in your blood (hypokalemia).
- Heart failure. Taking certain diabetes pills called TZDs with SOLIQUA 100/33 may cause heart failure in some people. This can happen even if you have never had heart failure or heart problems before. If you already have heart failure it may get worse while you take TZDs with SOLIQUA 100/33. Your healthcare provider should monitor you closely while you are taking TZDs with SOLIQUA 100/33. Tell your healthcare provider if you have any new or worse symptoms of heart failure including shortness of breath, swelling of your ankles or feet, or sudden weight gain. Treatment with TZDs and SOLIQUA 100/33 may need to be adjusted or stopped by your healthcare provider if you have new or worse heart failure.
- Gallbladder problems. Gallbladder problems have happened in some people who take SOLIQUA 100/33. Tell your healthcare provider right away if you get symptoms of gallbladder problems which may include:
- pain in your upper stomach (abdomen)
- yellowing of skin or eyes (jaundice)
- fever
- clay-colored stools
- pain in your upper stomach (abdomen)
- fever
- yellowing of skin or eyes (jaundice)
- clay-colored stools
- Food or liquid getting into the lungs during surgery or other procedures that use general anesthesia or deep sleepiness (deep sedation). SOLIQUA 100/33 may increase the chance of food getting into your lungs during surgery or other procedures. Tell all your healthcare providers that you are taking SOLIQUA 100/33 before you are scheduled to have a surgery or other procedures.
The most common side effects of SOLIQUA 100/33 include:
|
|
|
|
|
|
|
|
|
|
|
|
Talk to your healthcare provider about any side effect that bothers you or does not go away.
These are not all the possible side effects of SOLIQUA 100/33. Call your doctor for medical advice about side effects. You may report side effects to FDA at 1-800-FDA-1088 1-800-FDA-1088.
Click here for Full Prescribing Information for SOLIQUA 100/33.
Click here for information on Sharps Medical Waste Disposal.
Click here to learn more about Sanofi’s commitment to fighting counterfeit drugs.
*Eligibility Restrictions & Offer Terms:
Sanofi Insulins Co-pay Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is not valid for prescriptions covered by or submitted for reimbursement, in whole or in part, under Medicare, Medicaid, VA, DOD, TRICARE, similar federal or state programs, including any state pharmaceutical programs. If you have an Affordable Care (Health Care Exchange) plan, you may still be qualified to receive and use this savings card. Please note: the Federal Employees Health Benefits (FEHB) Program is not a federal or state government health care program for purposes of the savings program. Void where prohibited by law. For the duration of the program, eligible commercially insured patients pay no more than $35 per 30-day supply, up to 10 packs per fill; Offer valid for one fill every 30 days. Savings may vary depending on patients’ out-of-pocket costs. The Sanofi Insulins Co-pay Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Sanofi Insulins Co-pay Savings Program at (866) 255-8661 (866) 255-8661 (9:00 am-7:00 pm EST, Monday-Friday).
Insulins Valyou Savings Program: This savings program is not insurance. For a complete list of participating brands, products, and National Drug Codes (NDCs) Click Here. This offer is only valid for those who are uninsured or those who are insured by a prescription plan but are not using such insurance and will be paying the full retail price for the medication. Void where prohibited by law. For the duration of the program, eligible patients will pay $35 per 30-day supply. To pay $35 per 30-day supply, you must fill all your Sanofi Insulin prescriptions at the same time, together each month. The Savings Program applies to the cost of medication. There are other relevant costs associated with overall treatment. You may not submit claims for reimbursement to any third-party payor, including any government healthcare plan (e.g., Medicare, Medicaid, DOD, VA, TRICARE) or similar federal or state programs for Sanofi Insulin prescriptions when using this Program. You may not seek to have your out-of-pocket costs or the full retail price of the Sanofi Insulin count toward your deductible, true-out-of-pocket (TrOOP), maximum out-of-pocket (MOOP), or any other out-of-pocket caps associated with any insurance coverage. Sanofi reserves the right to rescind, revoke, terminate, or amend this offer, eligibility, and terms of use at any time without notice. Upon registration, patients will receive all program details. For questions regarding your eligibility or benefits, or if you wish to discontinue your participation, call the Insulins Valyou Savings Program at (833) 813-0190 (833) 813-0190 (9:00 am-7:00 pm EST, Monday-Friday).
References:
1. Lantus Prescribing Information.
2. Toujeo Prescribing Information.
If you are a patient experiencing problems with a Sanofi US product, please contact Sanofi US at 1-800-633-16101-800-633-1610.
The health information contained herein is provided for general education purposes only. Your healthcare professional is the single best source of information regarding your health. Please consult your healthcare professional if you have any questions about your health or treatment.





